Ventricular Systolic Dysfunction (HFrEF)
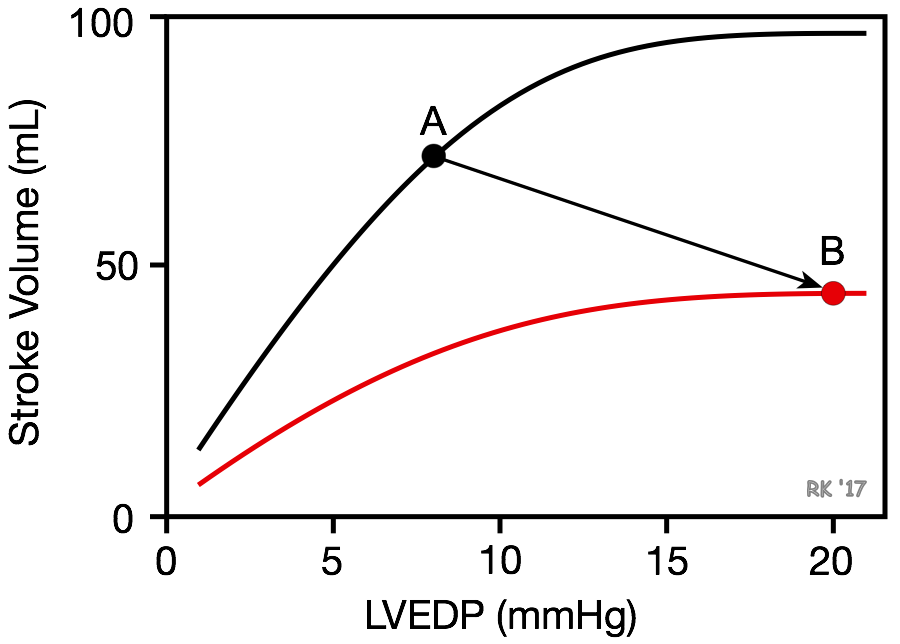 Systolic dysfunction refers to impaired ventricular contraction (loss of inotropy). In heart failure, this is most likely results from changes in the signal transduction mechanisms regulating cardiac excitation-contraction coupling. A decline in cardiac inotropy (i.e., decreased contractility) causes a downward shift in the Frank-Starling curve (red curve in figure). This results in a decrease in stroke volume and a compensatory increase in preload (often measured as ventricular end-diastolic pressure or pulmonary capillary wedge pressure) because of incomplete ventricular emptying. The reason preload increases as inotropy declines acutely is that the increased end-systolic volume is added to the venous return filling the ventricle. For example, if end-systolic volume is normally 50 ml of blood, and it is increased to 80 ml in failure, this extra residual volume added to the incoming venous return leads to an increase in end-diastolic volume and pressure. Therefore, there is a downward/rightward shift in the operating point on the Frank-Starling curve (point A to B). The increase in preload is a compensatory response that activates the Frank-Starling mechanism to help maintain stroke volume despite the loss of inotropy. If preload did not increase, the decline in stroke volume would be even greater for a given loss of inotropy.
Systolic dysfunction refers to impaired ventricular contraction (loss of inotropy). In heart failure, this is most likely results from changes in the signal transduction mechanisms regulating cardiac excitation-contraction coupling. A decline in cardiac inotropy (i.e., decreased contractility) causes a downward shift in the Frank-Starling curve (red curve in figure). This results in a decrease in stroke volume and a compensatory increase in preload (often measured as ventricular end-diastolic pressure or pulmonary capillary wedge pressure) because of incomplete ventricular emptying. The reason preload increases as inotropy declines acutely is that the increased end-systolic volume is added to the venous return filling the ventricle. For example, if end-systolic volume is normally 50 ml of blood, and it is increased to 80 ml in failure, this extra residual volume added to the incoming venous return leads to an increase in end-diastolic volume and pressure. Therefore, there is a downward/rightward shift in the operating point on the Frank-Starling curve (point A to B). The increase in preload is a compensatory response that activates the Frank-Starling mechanism to help maintain stroke volume despite the loss of inotropy. If preload did not increase, the decline in stroke volume would be even greater for a given loss of inotropy.
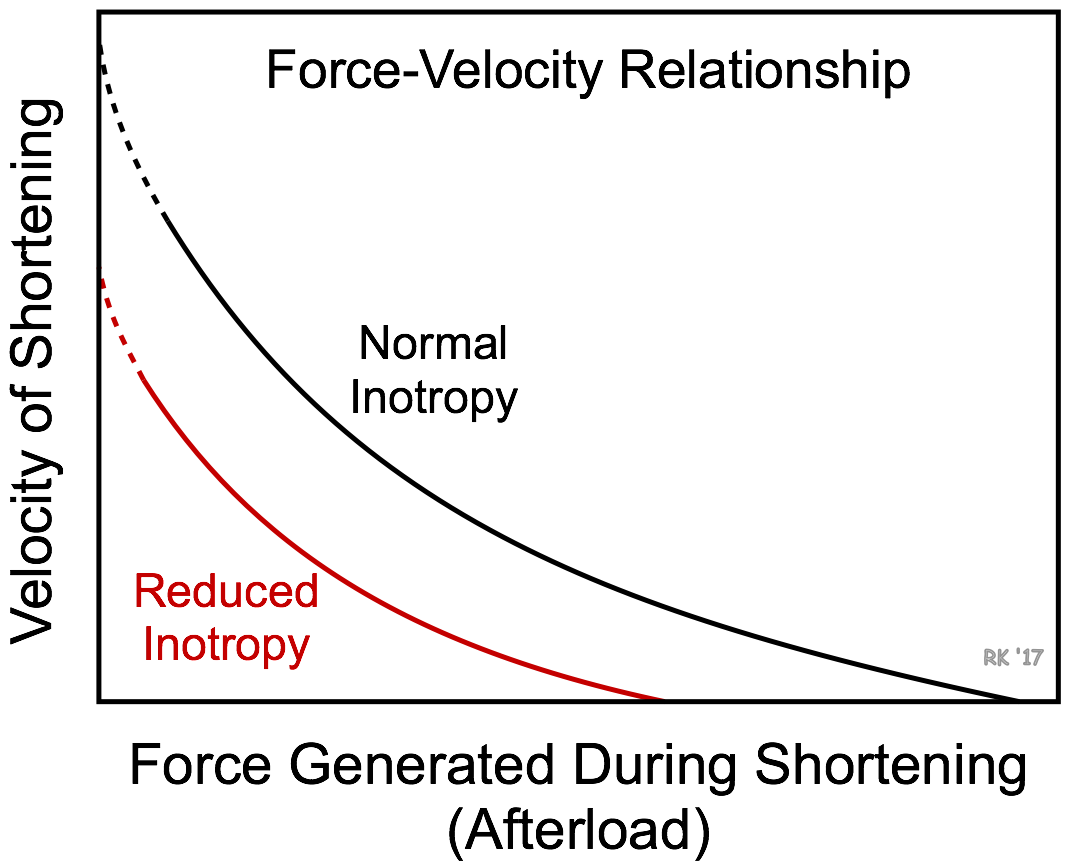 The force-velocity relationship for muscle fibers provides insight into why a loss of inotropy reduces stroke volume (see figure). The effects of afterload on muscle fiber shortening velocity is modified by the inotropic state of the muscle. Changes in inotropy cause parallel shifts in the force-velocity curve and changes in the Vmax (y-intercept) and the Fmax (x-intercept). Decreased inotropy causes the curve to shift down and to the left. At a given preload and afterload, a loss of inotropy results in a decrease in the shortening velocity of cardiac fibers and the velocity of ejection from the left ventricle. Because there is only a finite period of time available for ejection, reduced ejection velocity results in less blood ejected per stroke. The residual volume of blood within the ventricle (end-systolic volume) is increased because less blood is ejected.
The force-velocity relationship for muscle fibers provides insight into why a loss of inotropy reduces stroke volume (see figure). The effects of afterload on muscle fiber shortening velocity is modified by the inotropic state of the muscle. Changes in inotropy cause parallel shifts in the force-velocity curve and changes in the Vmax (y-intercept) and the Fmax (x-intercept). Decreased inotropy causes the curve to shift down and to the left. At a given preload and afterload, a loss of inotropy results in a decrease in the shortening velocity of cardiac fibers and the velocity of ejection from the left ventricle. Because there is only a finite period of time available for ejection, reduced ejection velocity results in less blood ejected per stroke. The residual volume of blood within the ventricle (end-systolic volume) is increased because less blood is ejected.
Acute and chronic heart failure with reduced ejection fraction (HFrEF)
Acute heart failure
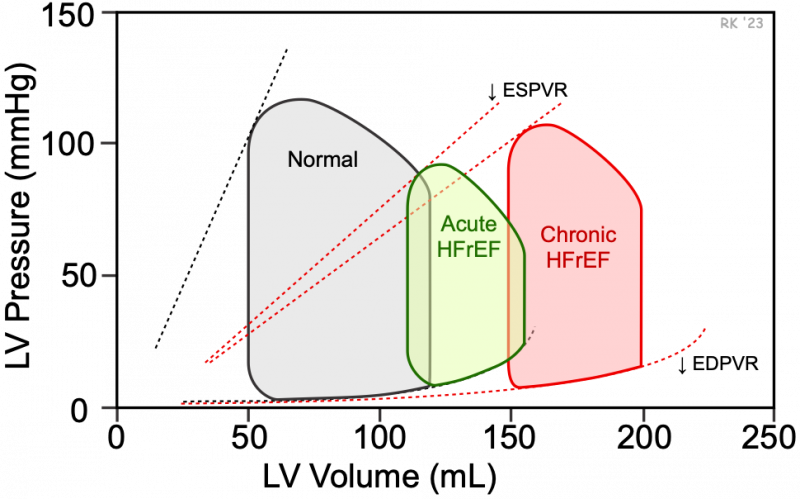
Heart failure caused by systolic dysfunction is referred to as heart failure with reduced ejection fraction (HFrEF). A loss of intrinsic inotropy on stroke volume and end-diastolic and end-systolic volumes is best depicted using ventricular pressure-volume loops (see figure). An acute loss in inotropy decreases the slope of the end-systolic pressure-volume relationship (ESPVR). This leads to an increase in end-systolic volume (green loop). There is also an increase in end-diastolic volume (compensatory increase in preload), but this increase is not as great as the increase in end-systolic volume. The increased filled volume leads to an increase in end-diastolic pressure (25 mmHg vs. 10 mmHg in the normal loop). Therefore, the net effect of acute failure is a decrease in stroke volume (shown as decreased width of the green loop). Because stroke volume decreases and end-diastolic volume increases, there is a substantial reduction in ejection fraction (EF), from 58 to 29% in this figure. Stroke work (area within the loop) is also decreased. Note that with acute HFrEF, there is no change in the end-diastolic pressure-volume relationship (EDPVR) or filling curve for the ventricle.
A rapid loss of inotropy can be caused by drug toxicity, electrolyte imbalance, acute myocardial ischemia, myocarditis, and prolonged ventricular tachycardia.
Chronic heart failure
Chronic HFrEF (red loop in above figure) is a condition whereby the heart responds over months and years to underlying diseases or conditions, such as valve disease, cardiomyopathies or ischemic heart disease. Long-term systolic dysfunction is associated with an increase in blood volume produced by neurohumoral compensatory mechanisms that contribute to increased ventricular filling and end-diastolic volume and pressure. Chronic volume overloading of the ventricle triggers structural remodeling, which leads to dilation of the ventricle. This results from new sarcomeres being added in-series to existing sarcomeres. These structural changes increase ventricular compliance and cause a shift in the end-diastolic pressure-volume relationship (EDPVR) downward and to the right (i.e., reduced slope). This enables the ventricle to accommodate an increased filled volume and blunts the increase in end-diastolic pressure and left atrial pressure, although chronic HFrEF is frequently associated with sufficiently elevated pulmonary pressures to cause pulmonary congestion and edema.
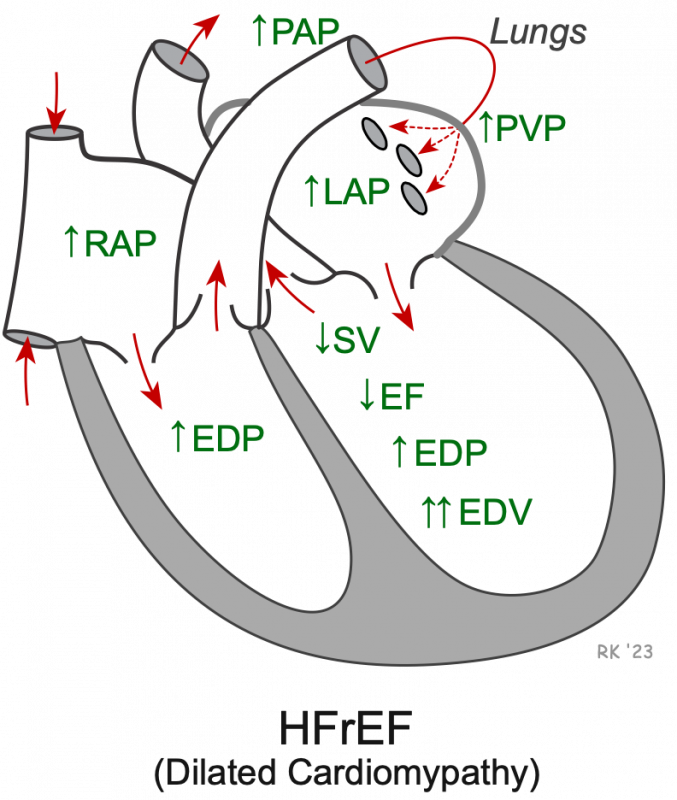 HFrEF is associated with a dilated, highly compliant, volume overloaded ventricle (increased end-diastolic volume, EDV). Despite the increased compliance, the end-diastolic pressure (EDP) is still elevated. This elevated pressure is transmitted back into the left atrium and thereby increases the left atrial pressure (LAP), the pulmonary capillary wedge pressure (PCWP), and the proximal pulmonary venous (PVP) and pulmonary artery (PAP) pressures. Stroke volume (SV) and EF are decreased. Right ventricular EDP and right atrial pressure (RAP) may also become elevated.
HFrEF is associated with a dilated, highly compliant, volume overloaded ventricle (increased end-diastolic volume, EDV). Despite the increased compliance, the end-diastolic pressure (EDP) is still elevated. This elevated pressure is transmitted back into the left atrium and thereby increases the left atrial pressure (LAP), the pulmonary capillary wedge pressure (PCWP), and the proximal pulmonary venous (PVP) and pulmonary artery (PAP) pressures. Stroke volume (SV) and EF are decreased. Right ventricular EDP and right atrial pressure (RAP) may also become elevated.
An important and deleterious consequence of systolic dysfunction is the increase in pulmonary pressures. This can lead to pulmonary congestion and edema. If the right ventricle is in systolic failure, the increase in end-diastolic pressure will be reflected backwards into the right atrium and systemic venous vasculature. This can lead to peripheral edema and ascites.
Treatment for systolic dysfunction (HFrEF) involves the use of inotropic drugs, afterload reducing drugs, venous dilators, and diuretics. Inotropic drugs include digoxin and drugs that stimulate the heart via beta-adrenoceptor activation or inhibition of cAMP-dependent phosphodiesterase. Afterload reducing drugs (e.g., arterial vasodilators) augment ventricular ejection by increasing the velocity of fiber shortening (see force-velocity relationship). Venous dilators and diuretics are used to reduce ventricular preload and venous pressures (pulmonary and systemic) rather than augmenting systolic function directly.
Revised 02/06/2024
 Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021)
Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021) Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)
Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)