Ventricular Diastolic Dysfunction (HFpEF)
Ventricular function highly depends on preload, as shown by the Frank-Starling relationship. Therefore, if ventricular filling (preload) is impaired, this will lead to a decrease in stroke volume. Diastolic dysfunction refers to changes in ventricular diastolic properties that adversely impact ventricular filling and stroke volume. About 50% of heart failure patients have diastolic dysfunction.
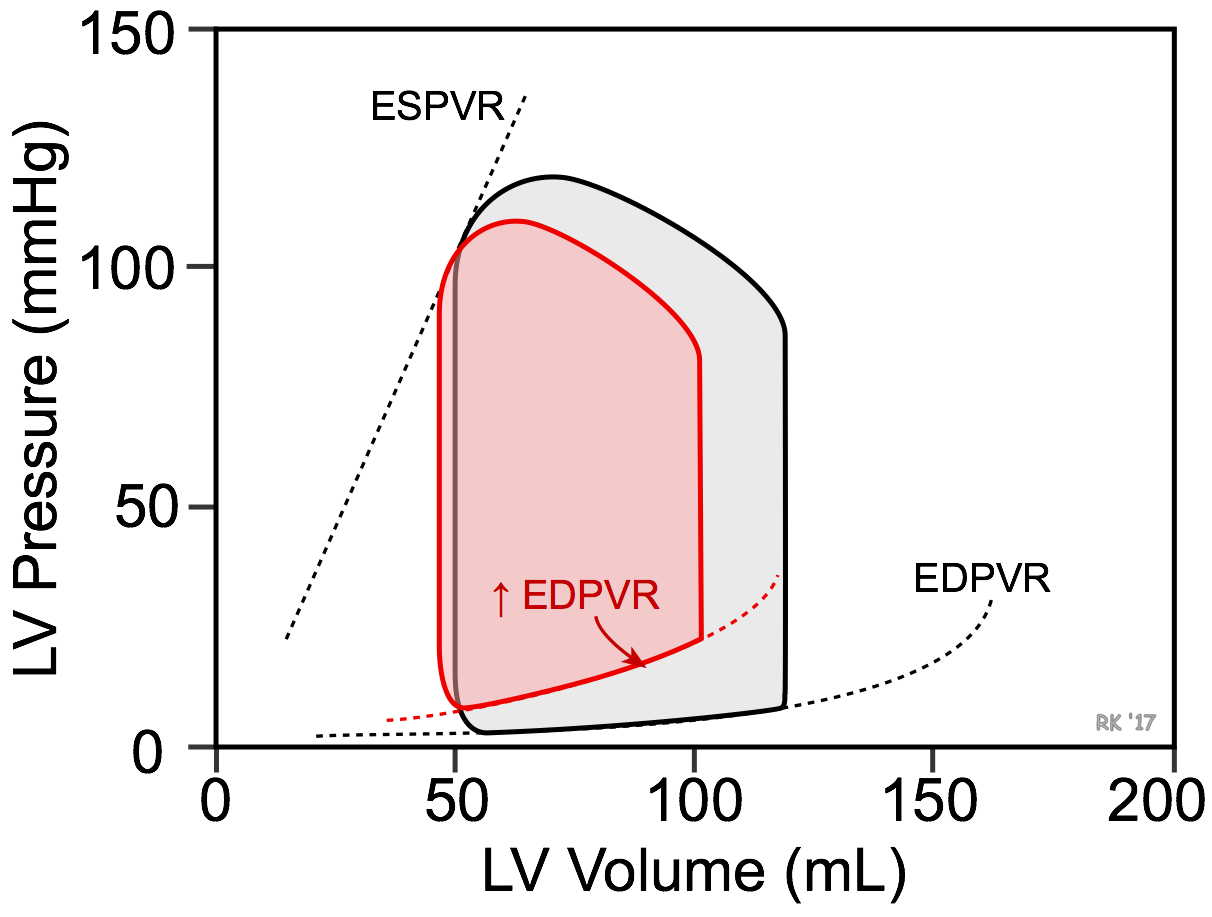 Ventricular filling (i.e., end-diastolic volume and hence sarcomere length) depends on the venous return and compliance of the ventricle during diastole. A reduction in ventricular compliance, as occurs in ventricular hypertrophy, increases the slope of the ventricular end-diastolic pressure-volume relationship (EDPVR) and results in less ventricular filling (decreased end-diastolic volume) and a greater end-diastolic pressure (elevated pulmonary capillary wedge pressures) as shown in the figure (red loop). Stroke volume, indicated by the width of the pressure-volume loop, decreases. Depending on the relative change in stroke volume and end-diastolic volume, there may or may not be a small decrease in ejection fraction (EF). The EF of the control loop in the figure is 58% compared to 54% in the red loop representing diastolic dysfunction. Heart failure caused by diastolic dysfunction is commonly referred to as heart failure with preserved ejection fraction (HFpEF; EF ≥ 50%). Because stroke volume is decreased, there will also be a decrease in ventricular stroke work.
Ventricular filling (i.e., end-diastolic volume and hence sarcomere length) depends on the venous return and compliance of the ventricle during diastole. A reduction in ventricular compliance, as occurs in ventricular hypertrophy, increases the slope of the ventricular end-diastolic pressure-volume relationship (EDPVR) and results in less ventricular filling (decreased end-diastolic volume) and a greater end-diastolic pressure (elevated pulmonary capillary wedge pressures) as shown in the figure (red loop). Stroke volume, indicated by the width of the pressure-volume loop, decreases. Depending on the relative change in stroke volume and end-diastolic volume, there may or may not be a small decrease in ejection fraction (EF). The EF of the control loop in the figure is 58% compared to 54% in the red loop representing diastolic dysfunction. Heart failure caused by diastolic dysfunction is commonly referred to as heart failure with preserved ejection fraction (HFpEF; EF ≥ 50%). Because stroke volume is decreased, there will also be a decrease in ventricular stroke work.
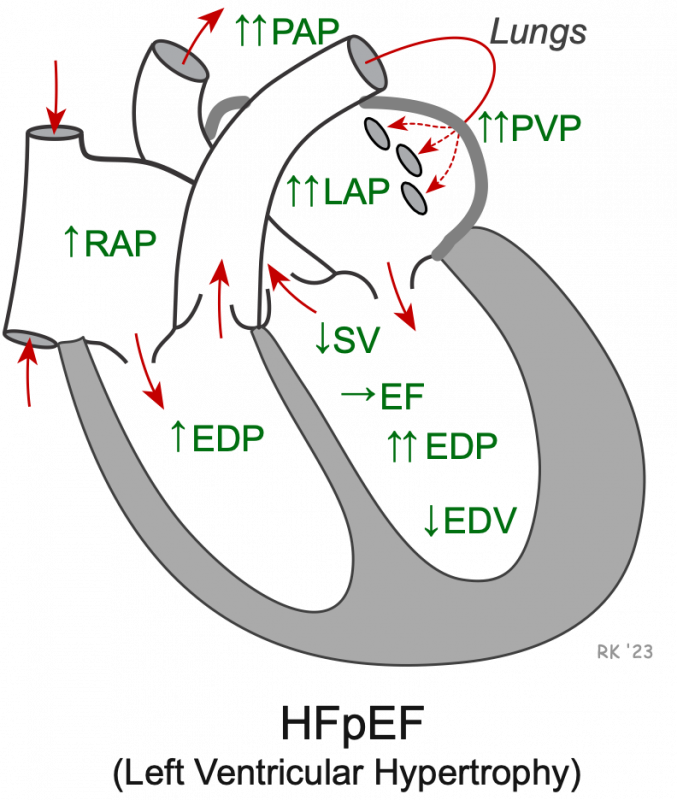 HFpEF most commonly results from left ventricular hypertrophy (reduced ventricular compliance). This can occur in response to chronic hypertension or aortic valve stenosis. A stiff ventricle leads to reduced filling (decreased end-diastolic volume, EDV) and elevated end-diastolic pressure (EDP). Reduce filling decreases stroke volume (SV), and the ratio of SV to EDV (i.e., EF) changes very little. The elevated left ventricular filling pressure is transmitted back into the left atrium and pulmonary vasculature, thereby increasing left atrial pressure (LAP), pulmonary venous pressure (PVP), and pulmonary arterial pressure (PAP). Right ventricular EDP and right atrial pressure (RAP) may also become elevated.
HFpEF most commonly results from left ventricular hypertrophy (reduced ventricular compliance). This can occur in response to chronic hypertension or aortic valve stenosis. A stiff ventricle leads to reduced filling (decreased end-diastolic volume, EDV) and elevated end-diastolic pressure (EDP). Reduce filling decreases stroke volume (SV), and the ratio of SV to EDV (i.e., EF) changes very little. The elevated left ventricular filling pressure is transmitted back into the left atrium and pulmonary vasculature, thereby increasing left atrial pressure (LAP), pulmonary venous pressure (PVP), and pulmonary arterial pressure (PAP). Right ventricular EDP and right atrial pressure (RAP) may also become elevated.
A second mechanism that is non-anatomical can also contribute to diastolic dysfunction: impaired ventricular relaxation (reduced lusitropy). Near the end of the cycle of excitation-contraction coupling in the myocyte, the sarcoplasmic reticulum actively sequesters Ca++ so that the concentration of Ca++ near troponin-C is reduced allowing the Ca++ to leave its binding sites on the troponin-C and permit disengagement of actin from myosin. This is a necessary step to achieve rapid and complete relaxation of the myocyte. If this mechanism is impaired (e.g., by reduced rate of Ca++ uptake by the sarcoplasmic reticulum), or by other mechanisms that contribute to myocyte relaxation, then the rate and perhaps the extent of relaxation are decreased. This will reduce the rate of ventricular filling, particularly during the phase of rapid filling.
An important and deleterious consequence of diastolic dysfunction is the rise in end-diastolic pressure. If the left ventricle is involved, then left atrial and pulmonary venous pressures will also rise. This can lead to pulmonary congestion and edema. If the right ventricle is in diastolic failure, the increase in end-diastolic pressure will be reflected backwards into the right atrium and systemic venous vessels. This can lead to peripheral edema and ascites. The rise in venous pressures also occurs because of an increase in blood volume caused by activation of the renin-angiotensin-aldosterone system, which leads to renal retention of sodium and water.
Revised 01/24/2024
 Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021)
Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021) Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)
Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)