Interdependent Effects of Preload, Afterload and Inotropy on Ventricular Pressure-Volume Loops
In the intact organism, changes in preload, afterload and inotropy are interdependent, meaning that changing one variable can alter the other two variables. The independent effects of preload, afterload and inotropy are described elsewhere (CLICK HERE). The following discussion uses left ventricular pressure-volume loops to illustrate the interdependent changes.
Interactions between Preload and Afterload at Constant Inotropy
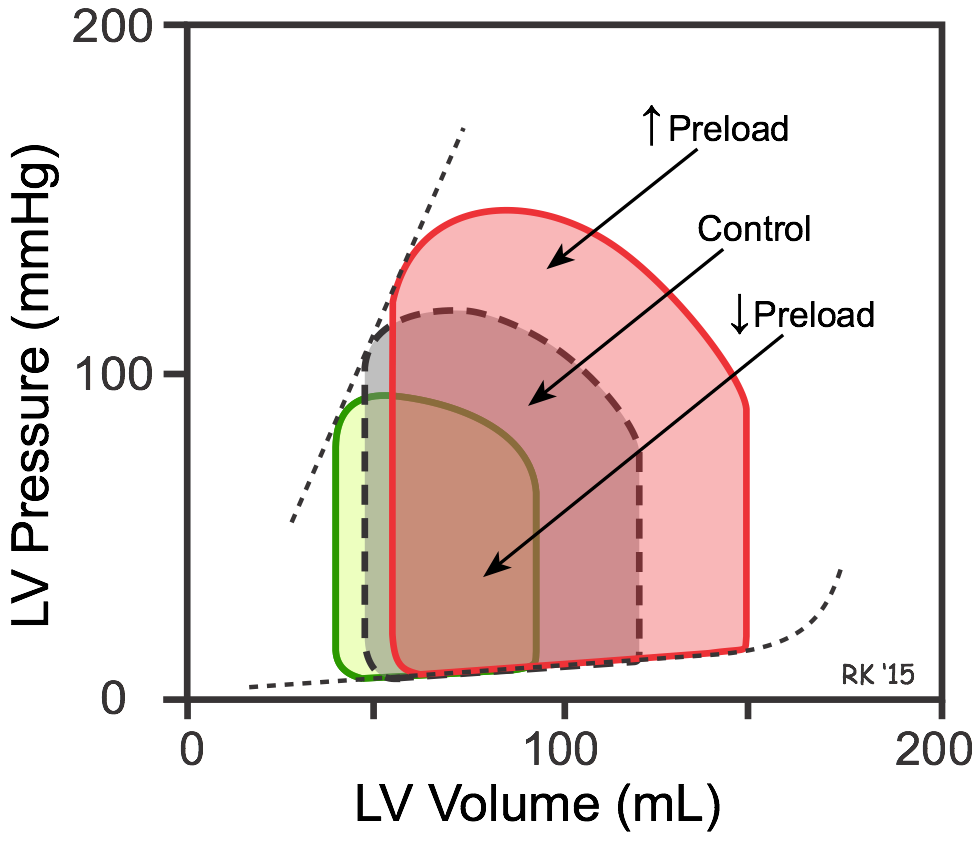 An increase in preload (end-diastolic volume represented by red loop in figure) leads to an increase in stroke volume (width of loop) because of the Frank-Starling mechanism. If afterload and inotropy do not change, then the end-systolic volume will not change, and the heart ejects all the extra blood that filled it (independent effect of preload). However, increased stroke volume leads to an increase in cardiac output and arterial pressure; therefore, the afterload on the ventricle increases. This partially offsets the increased stroke volume by increasing the end-systolic volume. This is because the increased afterload reduces the velocity of fiber shortening and therefore the ejection velocity (see force-velocity relationship). Conversely, a decrease in preload (green loop in figure) reduces stroke volume, but this reduction is partially offset by the decreased afterload (reduced aortic pressure) so that the end-systolic volume decreases slightly.
An increase in preload (end-diastolic volume represented by red loop in figure) leads to an increase in stroke volume (width of loop) because of the Frank-Starling mechanism. If afterload and inotropy do not change, then the end-systolic volume will not change, and the heart ejects all the extra blood that filled it (independent effect of preload). However, increased stroke volume leads to an increase in cardiac output and arterial pressure; therefore, the afterload on the ventricle increases. This partially offsets the increased stroke volume by increasing the end-systolic volume. This is because the increased afterload reduces the velocity of fiber shortening and therefore the ejection velocity (see force-velocity relationship). Conversely, a decrease in preload (green loop in figure) reduces stroke volume, but this reduction is partially offset by the decreased afterload (reduced aortic pressure) so that the end-systolic volume decreases slightly.
The above figure illustrating the effects of decreasing preload when afterload decreases, shows how the end-systolic pressure-volume relationship (ESPVR) is generated (dashed line connecting loop pressures at end-systole). The ESPVR relationship is generated by recording ventricular pressure-volume loops as the inferior vena cava is occluded. Vena cava occlusion decreases venous return to the heart, causing a progressive fall in end-diastolic volume (preload) over several beats. As the preload progressively decreases, the PV loop moves to the left and gets smaller. If a line is then drawn through the upper left corner of each loop (as shown for the three PV loops in the figure), the line represents the ESPVR, and both the slope and the x-intercept can be determined. It is important to construct the ESPVR relationship within a few seconds of occluding the vena cava (usually over several heart beats) to prevent sympathetic reflexes from increasing ventricular inotropy, which would increase the ESPVR slope. This is a valid experimental way to determine ESPVR because this relationship is relatively load-independent.
Interdependent Effects of Changes in Afterload
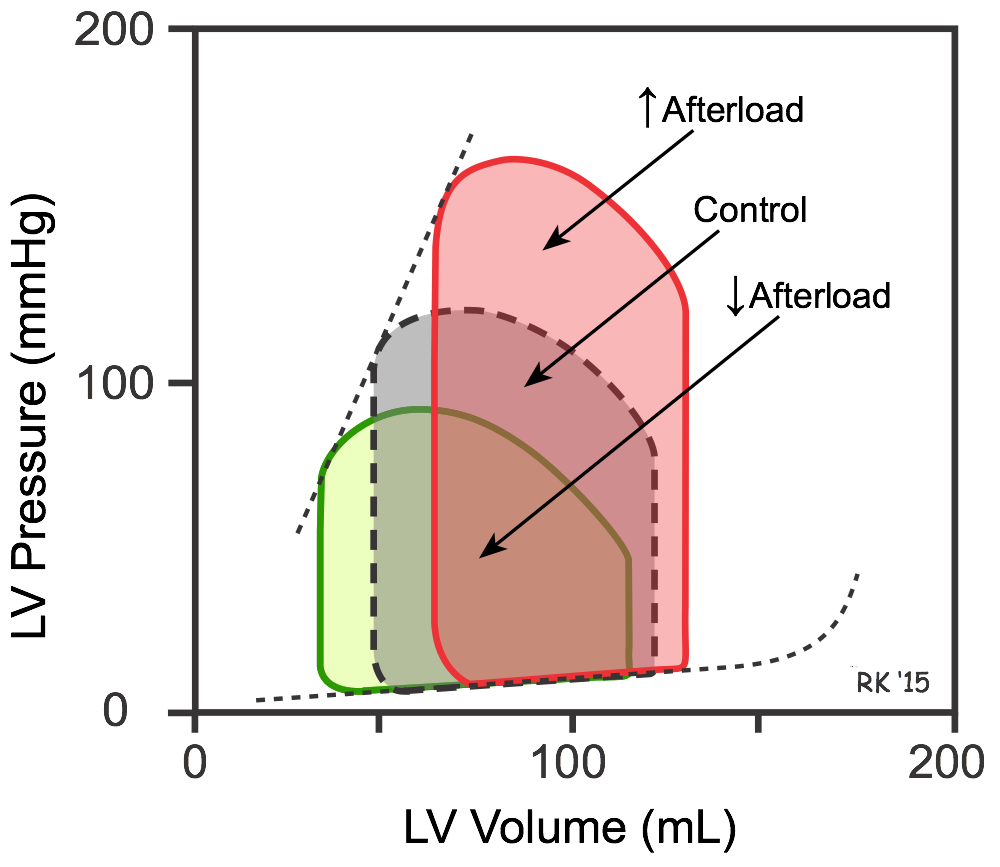 If afterload is increased (e.g., increasing aortic pressure by increasing systemic vascular resistance; red loop in figure), the stroke volume is reduced and the end-systolic volume increased. The increased end-systolic volume, however, leads to a secondary increase in end-diastolic volume because more blood is left inside the ventricle following ejection and this extra blood is added to the venous return, increasing ventricular filling. This secondary increase in preload enables the ventricle to contract with greater force (Frank-Starling mechanism), which partially offsets the reduction in stroke volume caused by the initial increase in afterload. Consequently, in a normal heart, changes in aortic pressure have relatively little effect on stroke volume. However, in heart failure patients in which the end-diastolic volume is already maximal, an increase in aortic pressure may lead to a significant reduction in stroke volume. If afterload (aortic pressure) is reduced (green loop in figure), the opposite changes occur - stroke volume increases because of the decrease in end-systolic volume, accompanied by a smaller reduction in end-diastolic volume. This is the basis for giving an arterial dilator to enhance cardiac output in heart failure patients.
If afterload is increased (e.g., increasing aortic pressure by increasing systemic vascular resistance; red loop in figure), the stroke volume is reduced and the end-systolic volume increased. The increased end-systolic volume, however, leads to a secondary increase in end-diastolic volume because more blood is left inside the ventricle following ejection and this extra blood is added to the venous return, increasing ventricular filling. This secondary increase in preload enables the ventricle to contract with greater force (Frank-Starling mechanism), which partially offsets the reduction in stroke volume caused by the initial increase in afterload. Consequently, in a normal heart, changes in aortic pressure have relatively little effect on stroke volume. However, in heart failure patients in which the end-diastolic volume is already maximal, an increase in aortic pressure may lead to a significant reduction in stroke volume. If afterload (aortic pressure) is reduced (green loop in figure), the opposite changes occur - stroke volume increases because of the decrease in end-systolic volume, accompanied by a smaller reduction in end-diastolic volume. This is the basis for giving an arterial dilator to enhance cardiac output in heart failure patients.
Interdependent Effects of Changes in Inotropy
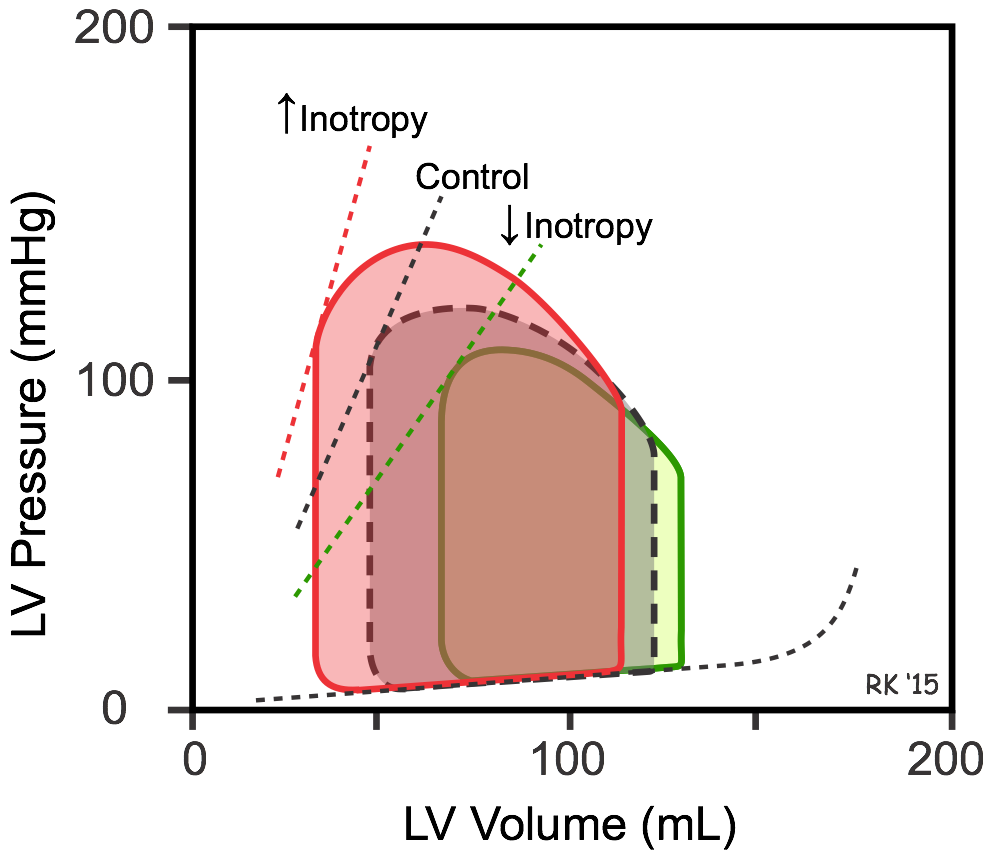 Increased inotropy (red loop in figure) increases the slope and shifts the end-systolic pressure-volume relationship (ESPVR) to the left, which permits the ventricle to generate more pressure at a given LV volume. Increased inotropy also increases the rate of pressure development and ejection velocity, which increases stroke volume and ejection fraction, and decreases end-systolic volume, as shown in the figure. With less blood remaining in the ventricle after ejection, the ventricle fills to a smaller end-diastolic volume during diastole, but this only partially offsets the reduction in end-systolic volume in the steady-state; therefore, stroke volume and ejection fraction increase. Increased cardiac output and arterial pressure increase ventricular afterload, which independently increases end-systolic volume; however, the response to increased afterload is overshadowed by the inotropic effects on end-systolic volume and stroke volume. A patient in acute heart failure caused by a loss of inotropy may be given a positive inotropic drug to increase stroke volume and to reduce ventricular preload, both of with are beneficial. Decreasing inotropy has the opposite effects (green loop in figure); namely, it increases end-systolic volume and decreases stroke volume and ejection fraction, accompanied by a small secondary increase in end-diastolic volume.
Increased inotropy (red loop in figure) increases the slope and shifts the end-systolic pressure-volume relationship (ESPVR) to the left, which permits the ventricle to generate more pressure at a given LV volume. Increased inotropy also increases the rate of pressure development and ejection velocity, which increases stroke volume and ejection fraction, and decreases end-systolic volume, as shown in the figure. With less blood remaining in the ventricle after ejection, the ventricle fills to a smaller end-diastolic volume during diastole, but this only partially offsets the reduction in end-systolic volume in the steady-state; therefore, stroke volume and ejection fraction increase. Increased cardiac output and arterial pressure increase ventricular afterload, which independently increases end-systolic volume; however, the response to increased afterload is overshadowed by the inotropic effects on end-systolic volume and stroke volume. A patient in acute heart failure caused by a loss of inotropy may be given a positive inotropic drug to increase stroke volume and to reduce ventricular preload, both of with are beneficial. Decreasing inotropy has the opposite effects (green loop in figure); namely, it increases end-systolic volume and decreases stroke volume and ejection fraction, accompanied by a small secondary increase in end-diastolic volume.
Interdependent Changes in Preload, Afterload and Inotropy during Exercise
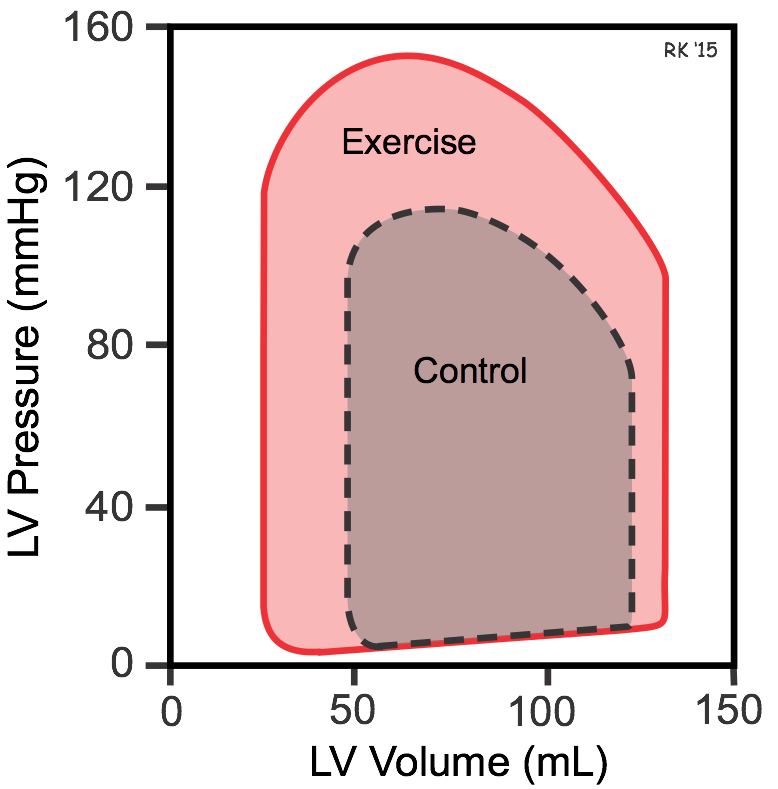 Exercise is a good example of how simultaneous changes in preload, afterload and inotropy affect ventricular pressures and volumes (red loop in figure). During moderate, upright, whole body exercise (e.g., running, bicycling) increased venous return to the heart by the muscle and respiratory pump systems may cause a small increase in end-diastolic volume (shown in figure); however, if heart rate increases to very high rates, reduced diastolic filling time can reduce end-diastolic volume. Sympathetic activation of the heart increases ventricular inotropy, which decreases end-systolic volume. The increased inotropy accompanied by enhanced venous return leads to an increase in stroke volume and ejection fraction, although these changes can be partially offset by very high heart rates. The increase in arterial pressure (increased ventricular afterload) that normally occurs during exercise diminishes the reduction in end-systolic volume; however, the large increase in inotropy is the dominate factor affecting end-systolic volume and stroke volume.
Exercise is a good example of how simultaneous changes in preload, afterload and inotropy affect ventricular pressures and volumes (red loop in figure). During moderate, upright, whole body exercise (e.g., running, bicycling) increased venous return to the heart by the muscle and respiratory pump systems may cause a small increase in end-diastolic volume (shown in figure); however, if heart rate increases to very high rates, reduced diastolic filling time can reduce end-diastolic volume. Sympathetic activation of the heart increases ventricular inotropy, which decreases end-systolic volume. The increased inotropy accompanied by enhanced venous return leads to an increase in stroke volume and ejection fraction, although these changes can be partially offset by very high heart rates. The increase in arterial pressure (increased ventricular afterload) that normally occurs during exercise diminishes the reduction in end-systolic volume; however, the large increase in inotropy is the dominate factor affecting end-systolic volume and stroke volume.
Revised 01/23/2023
 Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021)
Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021) Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)
Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)