Vascular Smooth Muscle Contraction and Relaxation
The contractile characteristics and the mechanisms that cause contraction of vascular smooth muscle (VSM) differ greatly from cardiac muscle. VSM undergoes slow, sustained tonic contractions, whereas cardiac muscle contractions are rapid and of relatively short duration (a few hundred milliseconds). While VSM contains actin and myosin, it does not have the regulatory protein troponin, as is found in the heart. Furthermore, the arrangement of actin and myosin in VSM is not organized into distinct bands as it is in cardiac muscle. This is not to imply that the contractile proteins of VSM are disorganized and not well-developed. They are actually highly organized and well-suited for their role in maintaining tonic contractions and reducing lumen diameter.
Contraction in VSM can be initiated by mechanical, electrical, and chemical stimuli. Passive stretching of VSM can cause contraction that originates from the smooth muscle itself and is, therefore, termed a myogenic response. Electrical depolarization of the VSM cell membrane also elicits contraction, most likely by opening voltage dependent calcium channels (L-type calcium channels), which causes an increase in the intracellular concentration of calcium. Finally, chemical stimuli such as norepinephrine, angiotensin II, vasopressin, endothelin-1, and thromboxane A2 can cause contraction. Each of these substances binds to specific receptors on the VSM cell or to receptors on the endothelium adjacent to the VSM, which then leads to VSM contraction. The mechanism of contraction (and relaxation) involves different signal transduction pathways.
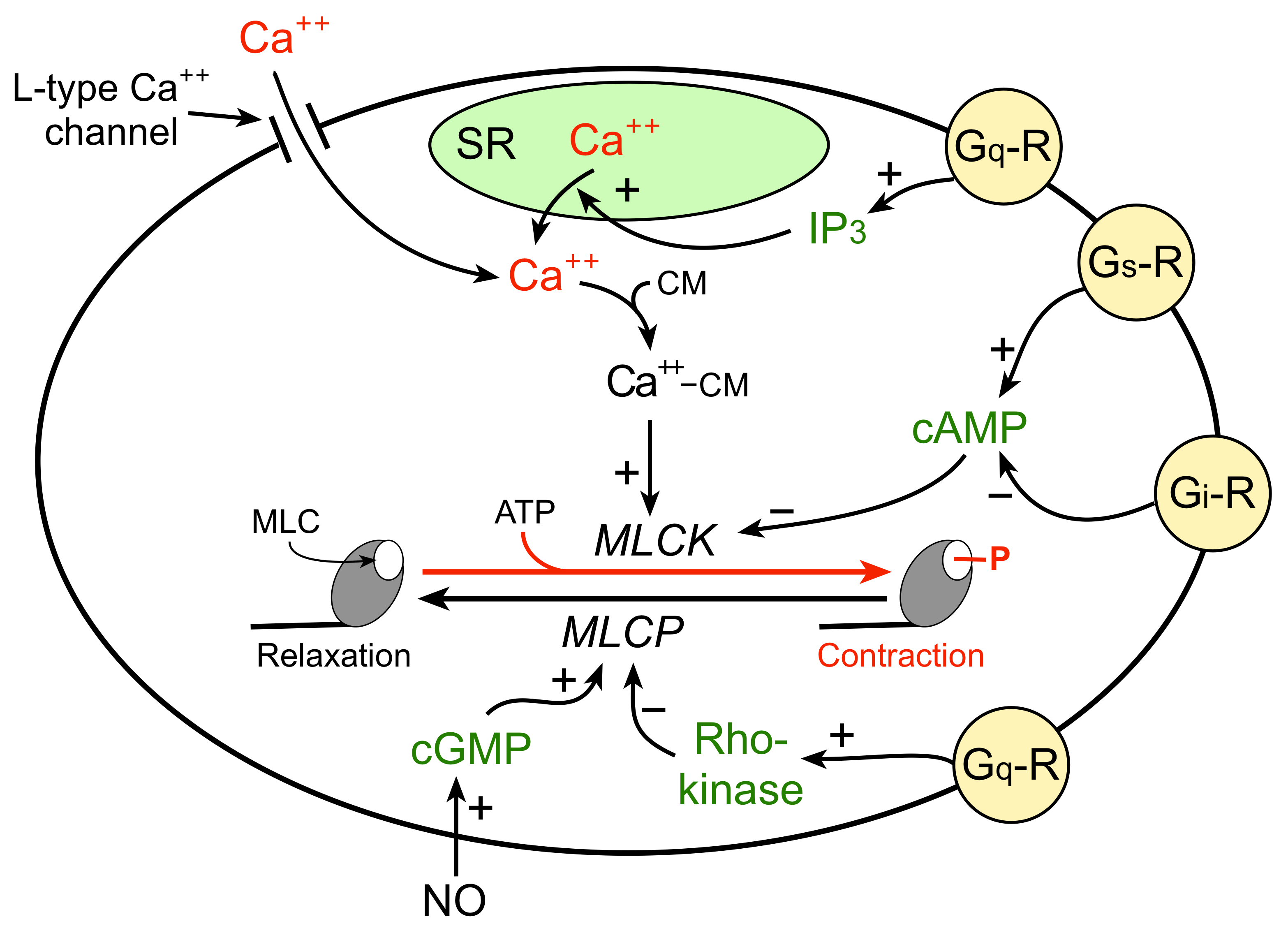
The mechanism by which an increase in intracellular calcium stimulates VSM contraction is illustrated in the figure to the right. An increase in free intracellular calcium can result from either increased flux of calcium into the cell through calcium channels or by release of calcium from internal stores (e.g., sarcoplasmic reticulum; SR). The free calcium binds to a special calcium binding protein called calmodulin. Calcium-calmodulin activates myosin light chain kinase (MLCK), an enzyme that phosphorylates myosin light chains (MLC) in the presence of ATP. Myosin light chains are found on the myosin heads. MLC phosphorylation leads to cross-bridge formation between the myosin heads and the actin filaments, and hence, smooth muscle contraction.
VSM relaxation occurs when there is reduced phosphorylation of MLC. This can result from 1) reduced release of calcium by the SR or reduced calcium entry into the cell, 2) inhibition of MLCK by increased intracellular concentration of cAMP, and 3) activation of myosin light chain phosphatase (MLCP), which dephosphorylates MLC. Therefore, vascular tone is determined by the degree of MLC phosphorylation, which depends on the relative activity of MLCK and MLCP.
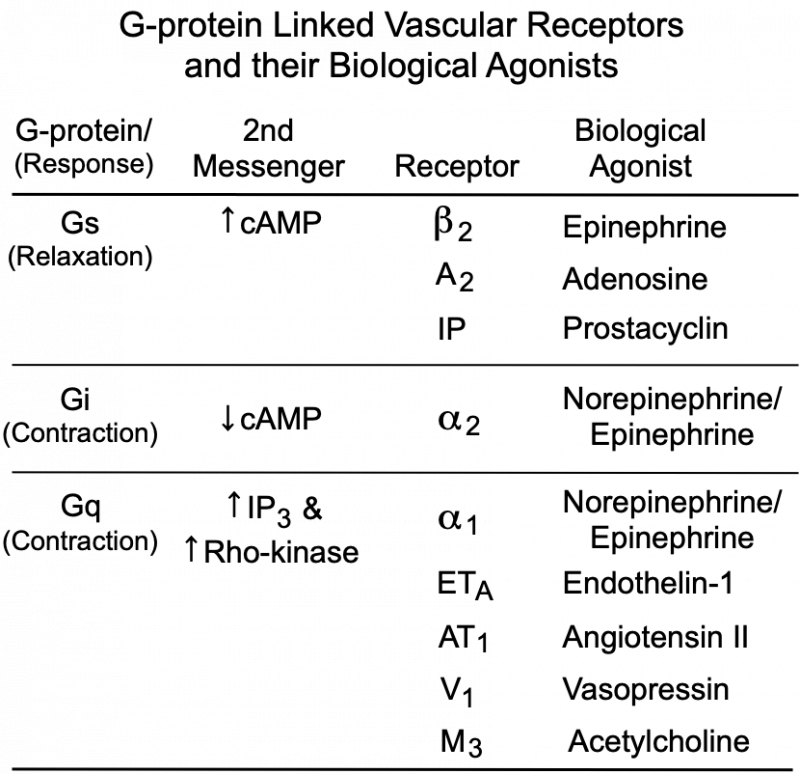 The degree of MLC phosphorylation (and therefore VSM contractile tone) is regulated by G-protein-couple signal transduction pathways and by nitric oxide activation of guanylyl cyclase and cGMP formation. As shown in the figure, Gq-protein activation by compounds such as norepinephrine (alpha1-adrenoceptors), angiotensin II (AT1 receptors), endothelin-1 (ETA receptors), acetylcholine (M3 receptors) and vasopressin (V1 receptors) stimulate SR release of calcium (IP3 mediated) and activates Rho-kinase, which inhibits MLC phosphatase (MLCP). Both mechanisms enhance VSM contraction. Gs-protein activation by compounds such as epinephrine (beta2-adrenoceptors), adenosine (A2 purinergic receptors) and prostacyclin (IP receptors) increase cAMP, which inhibits MLCK, reducing MLC phosphorylation and relaxing the VSM. Gi-protein activation by norepinephrine binding to alpha2-adrenoceptors elicits contraction by reducing cAMP, which increases the activity of MLCK.
The degree of MLC phosphorylation (and therefore VSM contractile tone) is regulated by G-protein-couple signal transduction pathways and by nitric oxide activation of guanylyl cyclase and cGMP formation. As shown in the figure, Gq-protein activation by compounds such as norepinephrine (alpha1-adrenoceptors), angiotensin II (AT1 receptors), endothelin-1 (ETA receptors), acetylcholine (M3 receptors) and vasopressin (V1 receptors) stimulate SR release of calcium (IP3 mediated) and activates Rho-kinase, which inhibits MLC phosphatase (MLCP). Both mechanisms enhance VSM contraction. Gs-protein activation by compounds such as epinephrine (beta2-adrenoceptors), adenosine (A2 purinergic receptors) and prostacyclin (IP receptors) increase cAMP, which inhibits MLCK, reducing MLC phosphorylation and relaxing the VSM. Gi-protein activation by norepinephrine binding to alpha2-adrenoceptors elicits contraction by reducing cAMP, which increases the activity of MLCK.
Intracellular calcium concentrations are also very important in regulating smooth muscle contraction. The concentration of intracellular calcium depends upon the balance between the calcium the enters the cells, the calcium that is released by intracellular storage sites (e.g., SR), and removal of calcium either back into storage sites or out of the cell. Calcium is re-sequestered by the SR by an ATP-dependent calcium pump. Calcium is removed from the cell to the external environment by either an ATP-dependent calcium pump or by the sodium-calcium exchanger.
Revised 8/25/2023
 Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021)
Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021) Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)
Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)