Sodium-Calcium Exchange in Cardiac Cells
Calcium is an important intracellular ion that regulates cardiac muscle and vascular smooth muscle electrical and mechanical activity. Intracellular calcium concentrations in both cardiac and vascular smooth muscle cells range from 10-7 to 10-5 M. Extracellular concentration of calcium is about 2 × 10-3 M (2 mM). Therefore, there is a chemical gradient for calcium to diffuse into the cell. Because cells have a negative resting membrane potential (about -90 mV in a cardiac myocyte), there is also an electrical force driving calcium into the cell. However, except during action potentials when the cell membrane permeability to calcium increases, there is little leakage of calcium into the cell. The calcium that enters the cell during action potentials (e.g., during depolarization of pacemaker and non-pacemaker cardiac cells) must be removed from the cell otherwise, an accumulation of calcium would lead to cellular dysfunction.
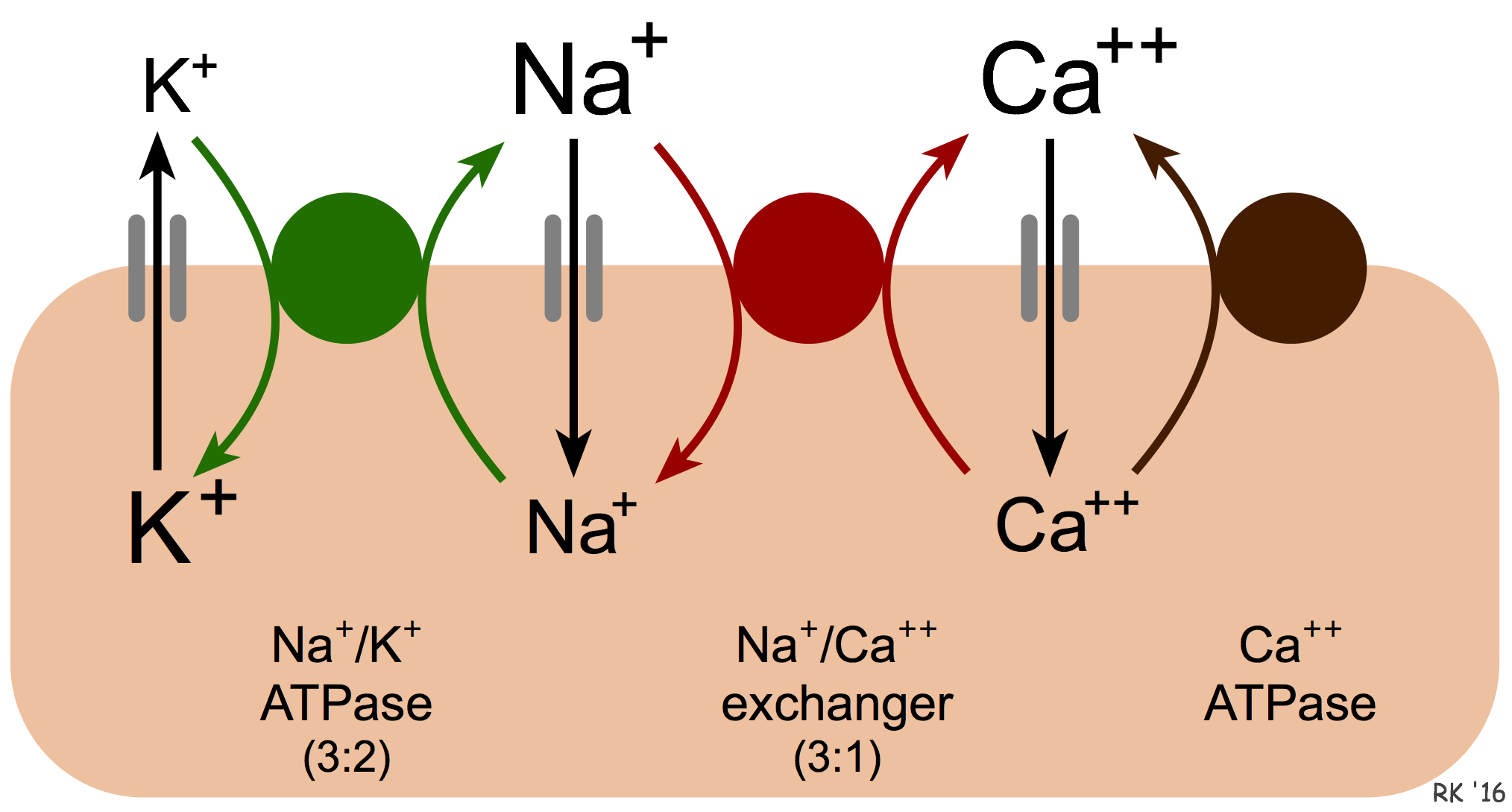 Calcium is removed from cells by two basic mechanisms. The first mechanism involves an ATP-dependent Ca++ pump that actively removes calcium from the cell (see figure). The second mechanism is the sodium-calcium exchanger (NCX). The exact mechanism by which this exchanger works is unclear. It is known that calcium and sodium can move in either direction across the sarcolemma. Furthermore, three sodium ions are exchanged for each calcium, therefore a small (few millivolts) electrogenic potential is generated by this exchanger. The direction of movement of these ions (either inward or outward) depends upon the membrane potential and the chemical gradient for the ions. When the membrane potential is negative (e.g., in resting cells), the exchanger transports Ca++ out as Na+ enters the cell. When the cell is depolarized and has a positive membrane potential, the exchanger works in the opposite direction (i.e., Na+ leaves and Ca++ enters the cell). Therefore, during ventricular systole, when the myocytes are depolarized, Ca++ enters the cell through this exchanger. In contrast, during ventricular diastole, when the cells are repolarized, Ca++ leaves the cell through this exchanger.
Calcium is removed from cells by two basic mechanisms. The first mechanism involves an ATP-dependent Ca++ pump that actively removes calcium from the cell (see figure). The second mechanism is the sodium-calcium exchanger (NCX). The exact mechanism by which this exchanger works is unclear. It is known that calcium and sodium can move in either direction across the sarcolemma. Furthermore, three sodium ions are exchanged for each calcium, therefore a small (few millivolts) electrogenic potential is generated by this exchanger. The direction of movement of these ions (either inward or outward) depends upon the membrane potential and the chemical gradient for the ions. When the membrane potential is negative (e.g., in resting cells), the exchanger transports Ca++ out as Na+ enters the cell. When the cell is depolarized and has a positive membrane potential, the exchanger works in the opposite direction (i.e., Na+ leaves and Ca++ enters the cell). Therefore, during ventricular systole, when the myocytes are depolarized, Ca++ enters the cell through this exchanger. In contrast, during ventricular diastole, when the cells are repolarized, Ca++ leaves the cell through this exchanger.
We also know that an increase in intracellular sodium concentration leads to an increase in intracellular calcium concentration through this exchange. This has important physiological implications. One example of this occurring is when the activity of the Na+/K+-ATPase pump is decreased. This energy requiring ATP-dependent pump transports sodium out of the cell and potassium into the cell. When the activity of this pump is reduced, for example, by cellular hypoxia (which causes ATP levels to fall) or by chemical inhibitors of this pump such as digoxin, then intracellular Na+ concentrations increase. One way to envision how this affects Ca++ exchange is that the increased intracellular Na+ reduces the concentration gradient of Na+ across the sarcolemma, which reduces the inward movement of Na+ down its concentration gradient via the exchanger. This reduces the outward movement and exchange of Ca++, which leads to an accumulation of intracellular calcium. This is the mechanism by which digoxin increases cardiac inotropy. Under hypoxic conditions, the enhanced calcium concentrations cannot increase inotropy because of the lack of ATP. The increased intracellular calcium (termed calcium overload) can damage mitochondria and alter cellular function.
Revised 01/24/2023
 Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021)
Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021) Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)
Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)