Cardiac Myocytes and Sarcomeres
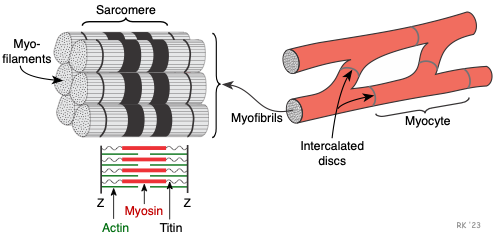 The cardiac myocyte is a specialized striated muscle cell that is approximately 25 μ in diameter and about 100 μ in length. They form a branching network of cells that are connected by intercalated discs. The myocyte is composed of bundles of myofibrils that contain myofilaments. The myofibrils have distinct, repeating microanatomical units, termed sarcomeres, which represent the basic contractile units of the myocyte (Figure 2). The sarcomere is defined as the region of myofilament structures between two Z-lines. The distance between Z-lines (i.e., sarcomere length) ranges from about 1.6 to 2.2 μ in human hearts. The sarcomere is composed of thick and thin filaments – myosin and actin, respectively, along with a titin that anchors the myosin to the Z-lines. Chemical and physical interactions between the actin and myosin cause the sarcomere length to shorten, and therefore the myocyte to contract during the process of excitation-contraction coupling. The interactions between actin and myosin serve as the basis for the sliding filament theory of muscle contraction.
The cardiac myocyte is a specialized striated muscle cell that is approximately 25 μ in diameter and about 100 μ in length. They form a branching network of cells that are connected by intercalated discs. The myocyte is composed of bundles of myofibrils that contain myofilaments. The myofibrils have distinct, repeating microanatomical units, termed sarcomeres, which represent the basic contractile units of the myocyte (Figure 2). The sarcomere is defined as the region of myofilament structures between two Z-lines. The distance between Z-lines (i.e., sarcomere length) ranges from about 1.6 to 2.2 μ in human hearts. The sarcomere is composed of thick and thin filaments – myosin and actin, respectively, along with a titin that anchors the myosin to the Z-lines. Chemical and physical interactions between the actin and myosin cause the sarcomere length to shorten, and therefore the myocyte to contract during the process of excitation-contraction coupling. The interactions between actin and myosin serve as the basis for the sliding filament theory of muscle contraction.
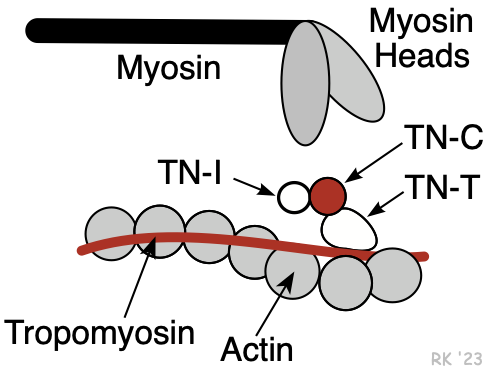 Myosin is a protein having a molecular weight of approximately 470,000 daltons. There are about 300 molecules of myosin per thick filament. Each myosin contains two heads that are the site of the myosin ATPase, an enzyme that hydrolyzes ATP required for actin and myosin cross bridge formation. These heads interact with a binding site on actin.
Myosin is a protein having a molecular weight of approximately 470,000 daltons. There are about 300 molecules of myosin per thick filament. Each myosin contains two heads that are the site of the myosin ATPase, an enzyme that hydrolyzes ATP required for actin and myosin cross bridge formation. These heads interact with a binding site on actin.
The thin filaments are composed of three different proteins: actin, tropomyosin, and troponin (Figure 2). Together, these are termed the regulatory protein complex. The actin is a globular protein arranged as a chain of repeating units, forming two strands of an alpha helix. Interdigitated between the actin strands are rod-shaped proteins, termed tropomyosin. There are 7 actin molecules per tropomyosin. Attached to the tropomyosin at regular intervals is the troponin complex (Figure 2), which comprises three subunits: troponin-T (TN-T), which attaches to the tropomyosin; troponin-C (TN-C), which serves as a binding site for Ca++ during excitation-contraction coupling (four Ca++ can bind per TN-C); and troponin-I (TN-I), which inhibits the myosin binding site on the actin. When Ca++ binds to TN-C, there is a conformational change in the troponin complex such that TN-I moves away from the myosin binding site on the actin, making it assessable to the myosin head. When Ca++ is removed from the TN-C, the troponin complex resumes its inactivated position, inhibiting myosin-actin binding. TN-I is important in clinical practice because it is used as a diagnostic marker for myocardial infarction (it is released into the circulation when myocytes die).
 Changes in sarcomere length are an important mechanism by which the heart regulates its force of contraction (see Frank-Starling relationship). As a myocyte is stretched (as occurs with increased ventricular preload), the sarcomeres within the myofibrils are also stretched. With increased sarcomere length, there is an increase in the force of contraction (i.e., tension development by the muscle fiber). For many years, it was thought that the major mechanism for the increased force generation was an increase in the overlap between actin and myosin, which made more sites available for hydrolysis of ATP by the myosin ATPase. This was an application of the sliding filament theory as described for striated skeletal muscle. While this may play a small role in the modulation of active tension development in cardiac muscle, there appear to be other mechanisms that are physiologically more important. Sarcomere lengths change little in cardiac muscle compared with skeletal muscle; nevertheless, small changes in cardiac sarcomere length can produce large changes in tension development. It is now known that stretching the sarcomere increases TN-C affinity for Ca++, which leads to increased tension development. This is referred to as length-dependent activation. For various structural and mechanical reasons, the sarcomere length in the cardiac myocytes rarely exceeds 2.2 μ.
Changes in sarcomere length are an important mechanism by which the heart regulates its force of contraction (see Frank-Starling relationship). As a myocyte is stretched (as occurs with increased ventricular preload), the sarcomeres within the myofibrils are also stretched. With increased sarcomere length, there is an increase in the force of contraction (i.e., tension development by the muscle fiber). For many years, it was thought that the major mechanism for the increased force generation was an increase in the overlap between actin and myosin, which made more sites available for hydrolysis of ATP by the myosin ATPase. This was an application of the sliding filament theory as described for striated skeletal muscle. While this may play a small role in the modulation of active tension development in cardiac muscle, there appear to be other mechanisms that are physiologically more important. Sarcomere lengths change little in cardiac muscle compared with skeletal muscle; nevertheless, small changes in cardiac sarcomere length can produce large changes in tension development. It is now known that stretching the sarcomere increases TN-C affinity for Ca++, which leads to increased tension development. This is referred to as length-dependent activation. For various structural and mechanical reasons, the sarcomere length in the cardiac myocytes rarely exceeds 2.2 μ.
Revised 01/23/2023
 Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021)
Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021) Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)
Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)