Vasopressin (Antidiuretic Hormone)
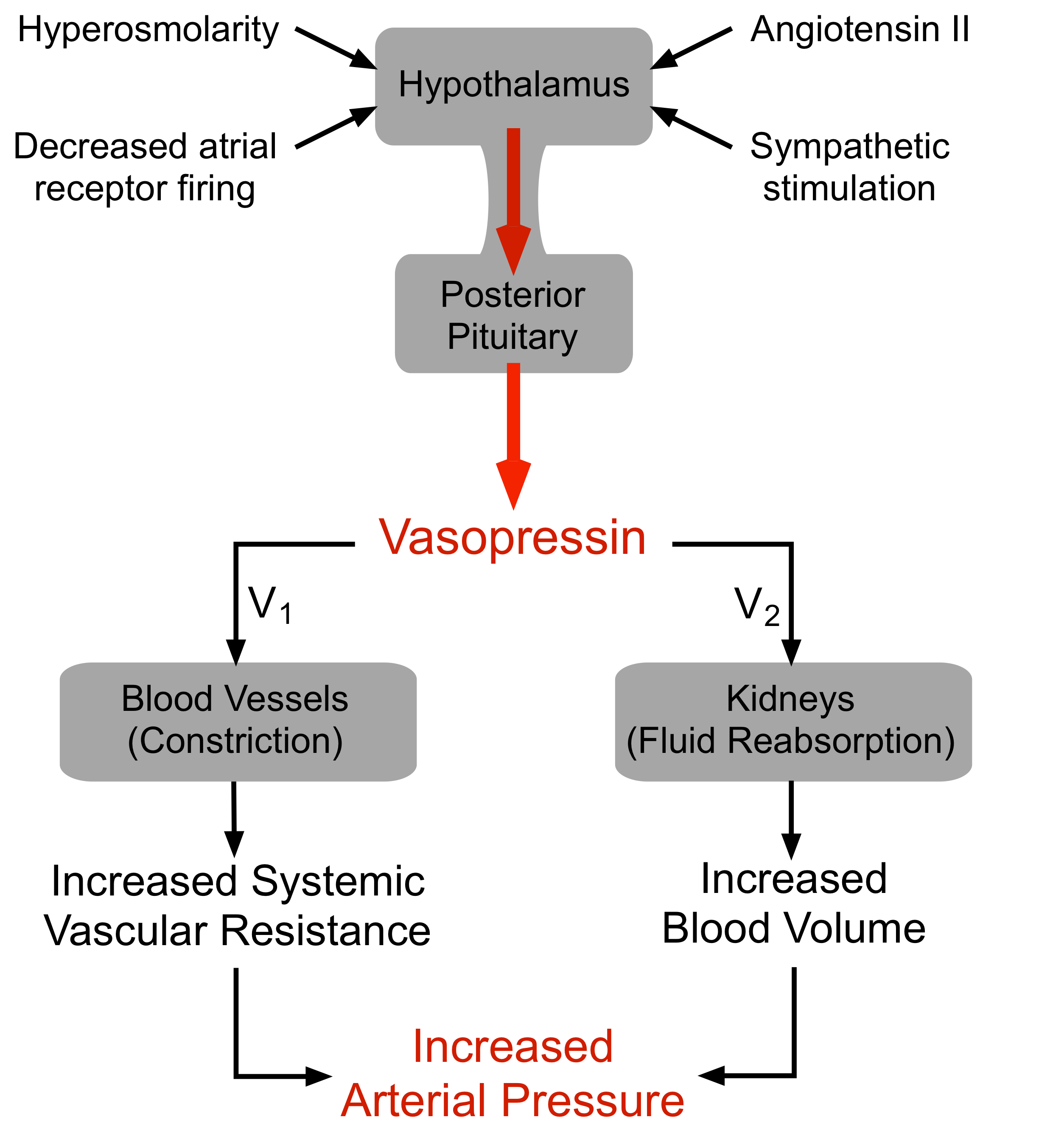 Vasopressin (arginine vasopressin, AVP; antidiuretic hormone, ADH) is a peptide hormone formed in the hypothalamus, then transported via axons to the posterior pituitary, which releases it into the blood.
Vasopressin (arginine vasopressin, AVP; antidiuretic hormone, ADH) is a peptide hormone formed in the hypothalamus, then transported via axons to the posterior pituitary, which releases it into the blood.
AVP has two principal sites of action, the kidney and blood vessels:
- The primary function of AVP in the body is to regulate extracellular fluid volume by regulating renal handling of water, although it is also a vasoconstrictor and pressor agent (hence, the name "vasopressin"). AVP acts on renal collecting ducts via V2 receptors to increase water permeability (cAMP-dependent mechanism), which leads to decreased urine formation (hence, the antidiuretic action of "antidiuretic hormone"). This increases blood volume, cardiac output, and arterial pressure.
- A secondary function of AVP is vasoconstriction. AVP binds to V1 receptors on vascular smooth muscle to cause vasoconstriction through the IP3 signal transduction pathway and Rho-kinase pathway, which increases arterial pressure; however, the normal physiological concentrations of AVP are below its vasoactive range. Studies have shown that in severe hypovolemic shock, when AVP release is very high, AVP contributes to the compensatory increase in systemic vascular resistance.
There are several mechanisms regulating the release of AVP, the most important of which are:
- Hypovolemia, as occurs during hemorrhage and dehydration, results in a decrease in atrial pressure. Specialized stretch receptors within the atrial walls and large veins (cardiopulmonary baroreceptors) entering the atria decrease their firing rate when there is a fall in atrial pressure. Afferent nerve fibers from these receptors synapse within the nucleus tractus solitarius of the medulla, which sends fibers to the hypothalamus, a region of the brain that controls AVP release by the pituitary. Atrial receptor (type A) firing normally inhibits the release of AVP by the posterior pituitary. With hypovolemia or decreased central venous pressure, the decreased firing of atrial stretch receptors leads to an increase in AVP release.
- Hypotension, which decreases arterial baroreceptor firing, leads to enhanced sympathetic activity that increases AVP release.
- Hypothalamic osmoreceptors sense extracellular osmolarity and stimulate AVP release when osmolarity rises, as occurs with dehydration.
- Angiotensin II receptors in a region of the hypothalamus regulate AVP release – an increase in angiotensin II simulates AVP release.
Heart failure is associated with what might be viewed as a paradoxical increase in AVP. Increased blood volume and atrial pressure associated with heart failure should decrease AVP secretion, but it does not. Sympathetic and renin-angiotensin system activation in heart failure may override the volume and low-pressure cardiovascular receptors (as well as the hypothalamic control of AVP release) and cause an increase in AVP secretion. Regardless of the mechanism, increased AVP during heart failure may contribute to the increase in systemic vascular resistance and the enhanced renal retention of fluid that accompanies heart failure.
AVP infusion is sometimes used in treating septic shock, a condition that can be caused by a bacterial infection in the blood and the release of bacterial endotoxins. Infusion of AVP increases systemic vascular resistance and elevates arterial pressure. Some studies have shown that low-dose infusions AVP (which are used in septic shock) also cause cerebral, pulmonary and renal dilation (mediated by endothelial release of nitric oxide) while constricting other vascular beds.
Revised 12/8/2022
 Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021)
Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021) Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)
Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)