Hemorrhagic Shock
Hemorrhagic shock is a clinical syndrome resulting from decreased blood volume (hypovolemia) caused by blood loss, which leads to reduced cardiac output and organ perfusion. Blood loss can be external (e.g., externally bleeding wound) or internal (e.g., internal bleeding caused by ruptured aortic aneurysm). The severity of hemorrhagic shock and associated symptoms depends on the volume of blood that is lost and how rapidly it is lost. A blood loss of <15% of total blood volume leads to only a small increase in heart rate and no significant change in arterial pressure. When blood loss is 15 to 40%, mean arterial and pulse pressures fall, and heart rate increases, with the magnitude of these changes being related to how much blood is lost. If the hemorrhage is stopped, the arterial pressure slowly recovers and the rapid heart rate declines as long-term compensatory mechanisms are activated to restore normal arterial pressure. The time for recovery is longer when there is a greater loss of blood. Resuscitation efforts, which include the administration of fluids to increase blood volume, can speed up this recovery. A greater than 40% blood loss is life-threatening, and clinical intervention is essential to ensure survival because prolonged, severe hypotension leads to organ failure and death.
Compensatory mechanisms
The reduction in blood volume during acute blood loss causes a fall in central venous pressure and cardiac filling. This leads to reduced cardiac output through the Frank-Starling mechanism, and reduced arterial pressure. The body has several compensatory mechanisms that become activated to restore arterial pressure and blood volume back to normal through negative feedback mechanisms, which include:
- Baroreceptor reflexes
- Chemoreceptor reflexes
- Circulating vasoconstrictors
- Renal reabsorption of sodium and water
- Activation of thirst mechanisms
- Reabsorption of tissue fluids
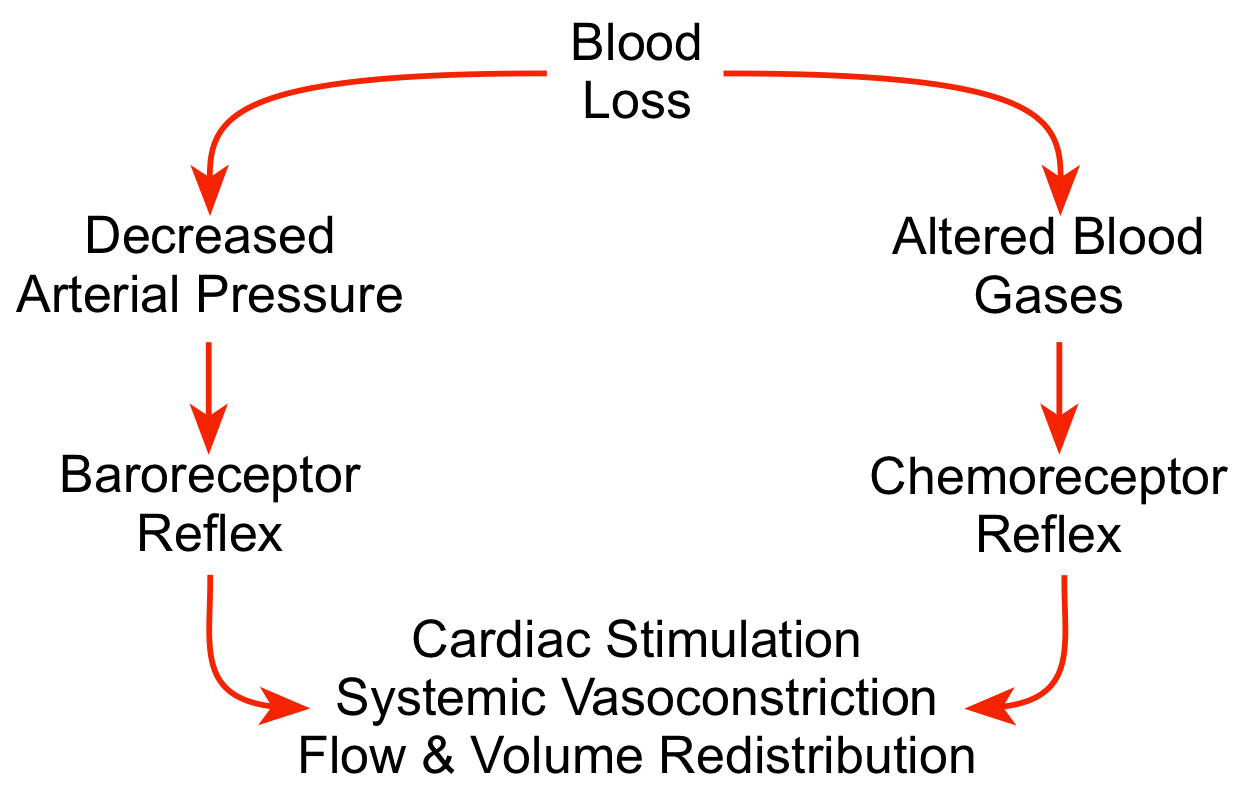 The body can quickly sense a fall in blood pressure through its arterial and cardiopulmonary baroreceptors, and then activate the sympathetic adrenergic system to stimulate the heart (increase heart rate and contractility) and constrict blood vessels (increase systemic vascular resistance). Sympathetic activation has little direct influence on brain and coronary blood vessels, so these circulations can benefit from the vasoconstriction that occurs in other organs (particularly in the gastrointestinal, skeletal muscle and renal circulations). Systemic vasoconstriction increases systemic vascular resistance and arterial pressure. Cardiac output is redistributed from less important organs to the brain and myocardium, both of which are critical for survival. Reduced organ blood flow caused by vasoconstriction and reduced arterial pressure leads to systemic acidosis that is sensed by chemoreceptors. The chemoreceptor reflex further activates the sympathetic adrenergic system, reinforcing the baroreceptor reflex. When the hypotension is very severe (e.g., mean arterial pressures <50 mmHg) and the brain becomes ischemic, this can produce a very intense sympathetic discharge that further reinforces the other autonomic reflexes.
The body can quickly sense a fall in blood pressure through its arterial and cardiopulmonary baroreceptors, and then activate the sympathetic adrenergic system to stimulate the heart (increase heart rate and contractility) and constrict blood vessels (increase systemic vascular resistance). Sympathetic activation has little direct influence on brain and coronary blood vessels, so these circulations can benefit from the vasoconstriction that occurs in other organs (particularly in the gastrointestinal, skeletal muscle and renal circulations). Systemic vasoconstriction increases systemic vascular resistance and arterial pressure. Cardiac output is redistributed from less important organs to the brain and myocardium, both of which are critical for survival. Reduced organ blood flow caused by vasoconstriction and reduced arterial pressure leads to systemic acidosis that is sensed by chemoreceptors. The chemoreceptor reflex further activates the sympathetic adrenergic system, reinforcing the baroreceptor reflex. When the hypotension is very severe (e.g., mean arterial pressures <50 mmHg) and the brain becomes ischemic, this can produce a very intense sympathetic discharge that further reinforces the other autonomic reflexes.
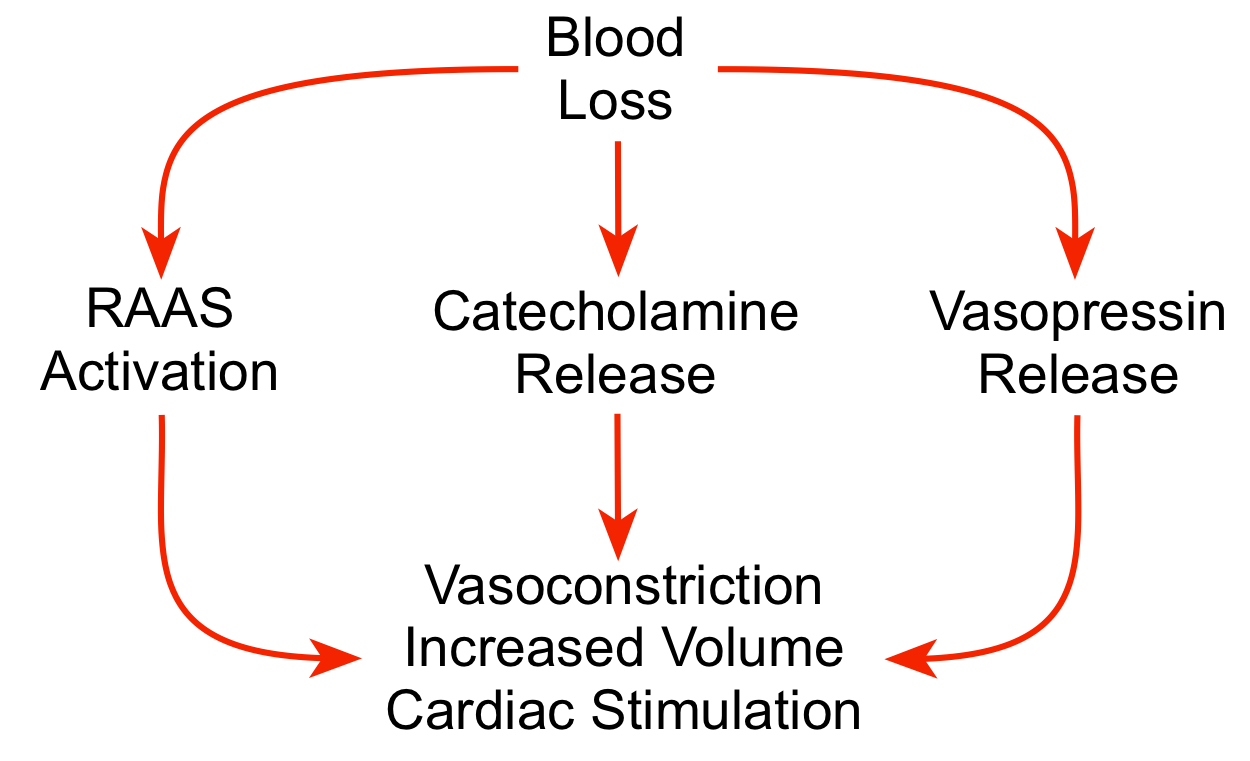 The combined effects of arterial hypotension and sympathetic activation lead to the activation of humoral compensatory mechanisms. Sympathetic stimulation of the adrenal glands stimulates the release of catecholamines into the blood, which reinforces the effects of sympathetic activation on the heart and vasculature. The kidneys release renin following hemorrhage, leading to increased circulating levels of angiotensin II and aldosterone. This causes vascular constriction, enhanced sympathetic activity, stimulation of vasopressin release, activation of thirst mechanisms, and, importantly, increased renal reabsorption of sodium and water to increase blood volume. This renal mechanism is important in the long-term recovery from blood loss.
The combined effects of arterial hypotension and sympathetic activation lead to the activation of humoral compensatory mechanisms. Sympathetic stimulation of the adrenal glands stimulates the release of catecholamines into the blood, which reinforces the effects of sympathetic activation on the heart and vasculature. The kidneys release renin following hemorrhage, leading to increased circulating levels of angiotensin II and aldosterone. This causes vascular constriction, enhanced sympathetic activity, stimulation of vasopressin release, activation of thirst mechanisms, and, importantly, increased renal reabsorption of sodium and water to increase blood volume. This renal mechanism is important in the long-term recovery from blood loss.
Hypotension, combined with constriction of precapillary resistance vessels (small arteries and arterioles), causes a fall in capillary hydrostatic pressure. This pressure normally drives filtration of fluid from the blood, across the capillary endothelium, and into the interstitial space. When capillary hydrostatic pressure is reduced, less fluid leaves the capillaries, and when the pressure falls sufficiently low as occurs following moderate-to-severe blood loss, net reabsorption of fluid can occur from the tissue interstitium back into the capillary plasma. Although this reabsorbed fluid does not contain cells, it contains electrolytes and some protein, and therefore increases the plasma volume. This reabsorbed fluid leads to hemodilution of the blood; therefore, red cell hematocrit falls in response to this fluid shift. This mechanism can cause up to 1 liter/hour of fluid to be withdrawn from interstitial spaces back into the plasma.
Decompensatory mechanisms
If compensatory mechanisms cannot restore arterial pressure, irreversible shock can occur. Circulatory decompensation is defined as failure of neurohumoral compensatory mechanisms and resuscitation to maintain a critical level of arterial pressure sufficient to perfuse vital organs, which leads to irreversible shock and death. This is observed when prolonged shock leads to resuscitation failure, even when blood volume is completely restored by blood transfusions. Many mechanisms can contribute to decompensation, some of which are summarized below:
Cardiogenic shock
- Impaired coronary blood flow resulting from hypotension causes myocardial hypoxia and acidosis, which depress cardiac function and cause arrhythmias.
Sympathetic escape
- Reduced organ flow leads to an accumulation of tissue metabolic vasodilator substances, which impairs sympathetic-mediated vasoconstriction and leads to loss of vascular tone, progressive hypotension and further organ hypoperfusion.
- Loss of precapillary vascular tone in severely hypoxic tissues increases capillary hydrostatic pressure and capillary fluid filtration, which reduces plasma volume.
Cerebral ischemia/hypoxia
- Loss of sympathetic outflow from a hypoxic medulla leads to vasodilation, which further reduces arterial pressure and cerebral perfusion.
Metabolic acidosis
- Reduced tissue perfusion and activation of anaerobic metabolism leads to metabolic acidosis, which depresses cardiac muscle and vascular smooth muscle contraction and therefore leads to further decreases in arterial pressure.
Rheological factors
- Reduced microcirculatory flow velocity causes blood viscosity within tissues to increase, which further reduces perfusion.
- Plugging of the microcirculation by leukocytes and platelets, and intravascular coagulation that reduce organ perfusion.
Systemic inflammatory response
- Endotoxins released into the systemic circulation from the ischemic gastrointestinal tract lead to cytokine production, and enhanced formation of nitric oxide and oxygen free radicals, which cause vasodilation, cardiac depression, and organ injury.
You can view a 33-minute YouTube lecture on Hemorrhage Shock.
Revised 11/03/2023
 Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021)
Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021) Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)
Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)