Cardiac Signal Transduction Mechanisms (G-Protein-Linked)
There are several major signal transduction mechanisms found in cells of the cardiovascular system, the most important being the G-protein and nitric oxide-cyclic GMP pathways. Described below are the G-protein-coupled pathways found in the heart. Signal transduction mechanisms regulating vascular smooth muscle contraction and relaxation are found elsewhere (Click Here).
Gs-Protein and Gi-Protein Coupled Signal Transduction
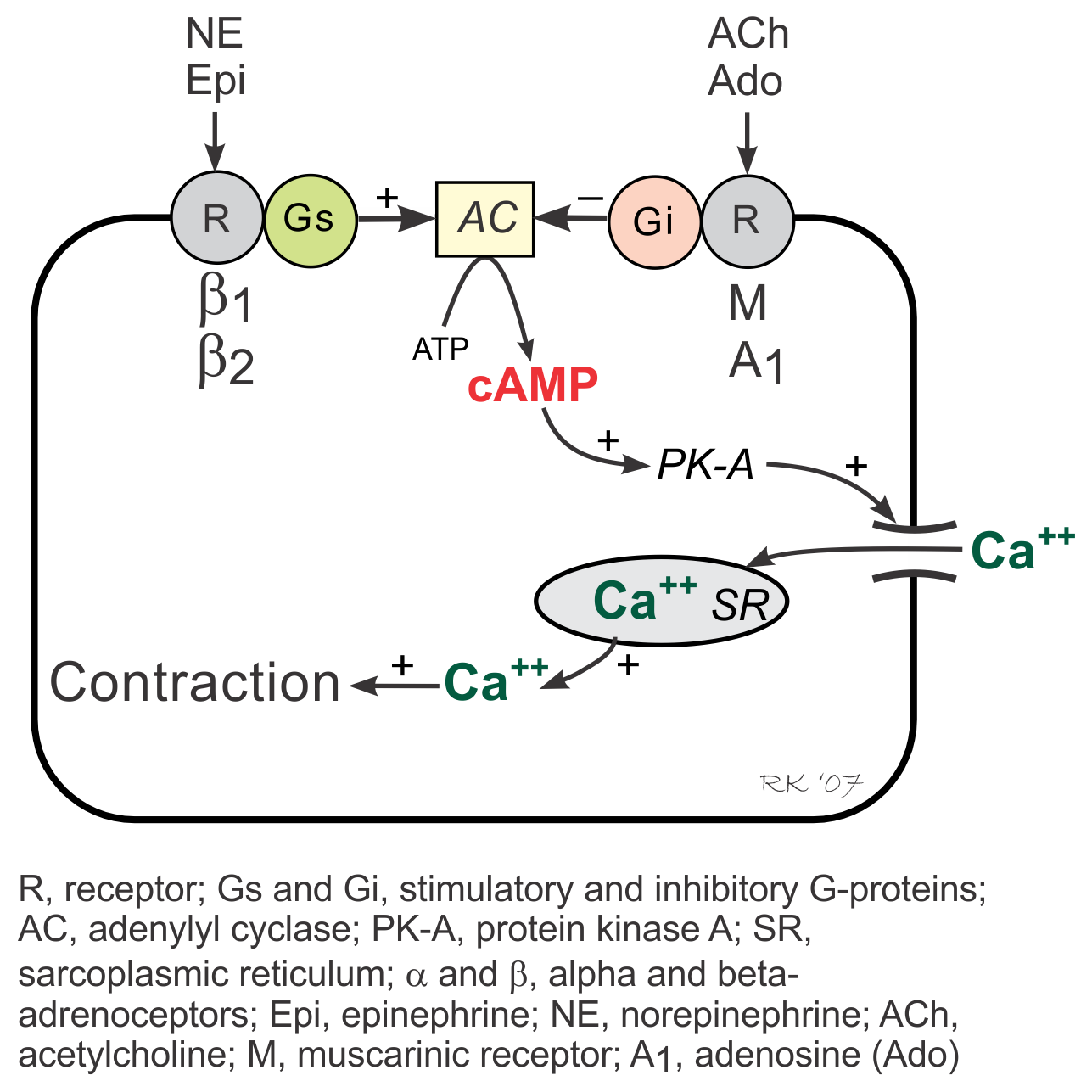 G-proteins are linked to an enzyme, adenylyl cyclase, that dephosphorylates ATP to form cyclic AMP (cAMP). Gs-protein (stimulatory G-protein) activation (e.g., via β-adrenoceptors) increases cAMP by activating adenylyl cyclase. cAMP then activates PK-A (cAMP stimulated protein kinase) and causes increased cellular influx of Ca++ by phosphorylating and activating membrane L-type calcium channels, and by enhancing release of Ca++ by the sarcoplasmic reticulum in the heart. These and other intracellular events increase inotropy (muscle contractility), chronotropy (heart rate), dromotropy (velocity of electrical conduction) and lusitropy (relaxation rate).
G-proteins are linked to an enzyme, adenylyl cyclase, that dephosphorylates ATP to form cyclic AMP (cAMP). Gs-protein (stimulatory G-protein) activation (e.g., via β-adrenoceptors) increases cAMP by activating adenylyl cyclase. cAMP then activates PK-A (cAMP stimulated protein kinase) and causes increased cellular influx of Ca++ by phosphorylating and activating membrane L-type calcium channels, and by enhancing release of Ca++ by the sarcoplasmic reticulum in the heart. These and other intracellular events increase inotropy (muscle contractility), chronotropy (heart rate), dromotropy (velocity of electrical conduction) and lusitropy (relaxation rate).
Activation of Gi-proteins (inhibitory G-protein), for example by adenosine and muscarinic agonists (e.g., acetylcholine) binding to their receptors, decreases cAMP (through adenylyl cyclase inhibition), inactivates PK-A, decreases Ca++ entry into the cell and release by the sarcoplasmic reticulum, and increases outward, hyperpolarizing K+ currents.
Gi-protein activation produces effects that are opposite to those elicited by Gs-protein activation. Because Gi-protein effects are primarily found in the SA node and AV node where there are an abundance of Gi-protein coupled receptors, activation of this pathway leads to a decrease in sinus rate and AV nodal conduction velocity with minimal effects on muscle contractility, particularly in the ventricles. In contrast, Gs-protein strongly stimulates muscle contraction, besides having nodal effects.
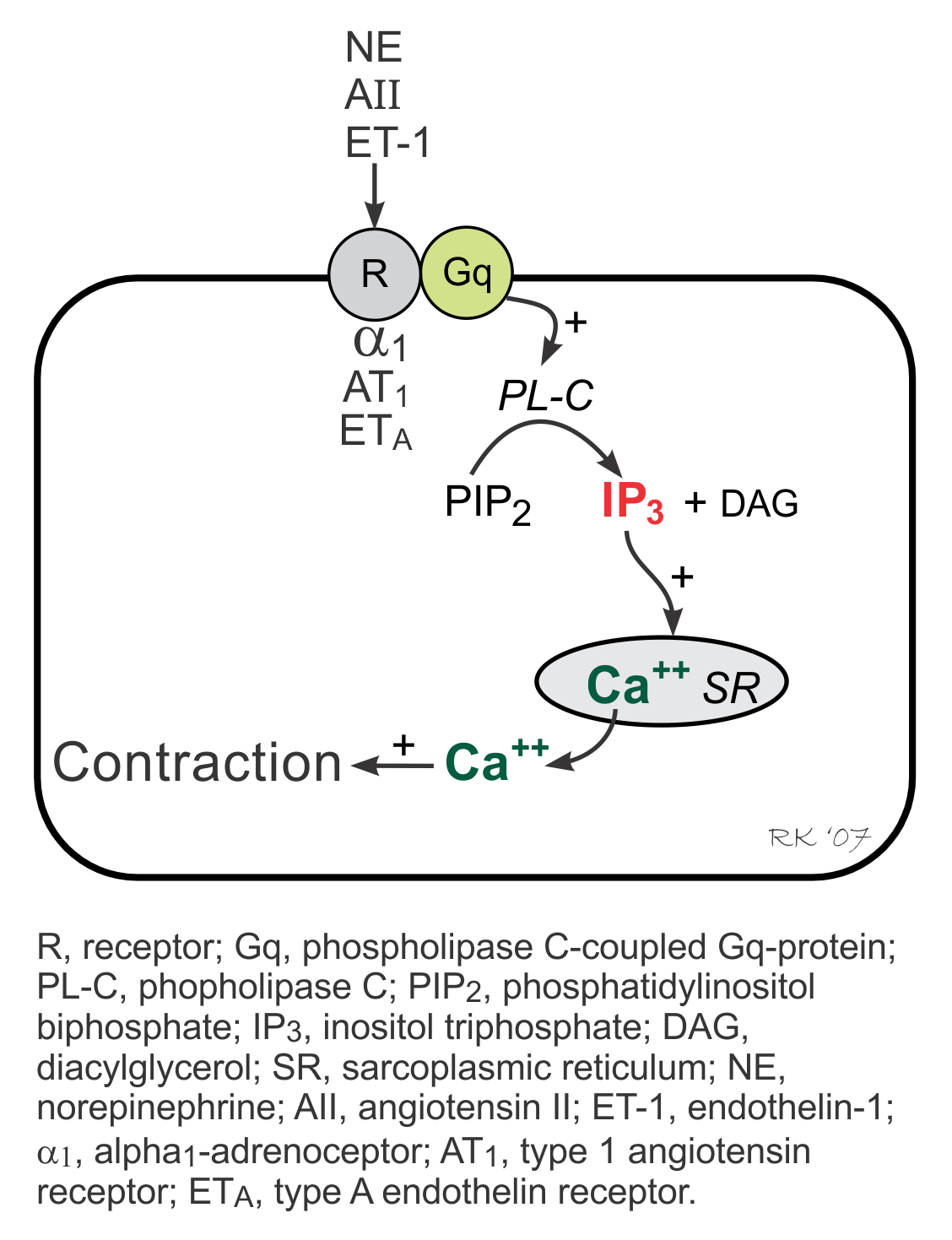 Gq-Protein and IP3- Coupled Signal Transduction
Gq-Protein and IP3- Coupled Signal Transduction
The IP3 pathway is linked to activation of α1-adrenoceptors, angiotensin II (AII) receptors, and endothelin-1 (ET-1) receptors and therefore is stimulated by alpha-agonists, angiotensin II and endothelin-1. These receptors are coupled to a phospholipase C (PL-C)-coupled Gq-protein, which when activated, stimulates the formation of inositol triphosphate (IP3) from phosphatidylinositol biphosphate (PIP2). Increased IP3 stimulates Ca++ release by the sarcoplasmic reticulum in the heart, increasing inotropy as one of its actions. Gq-protein coupled responses in the heart, however, are relatively minor compared to Gs-protein coupled responses.
Altered Signal Transduction in Heart Disease
Altered signal transduction mechanisms have a significant role in the loss of inotropy in heart failure. For example, desensitization of β1-adrenoceptors in the heart decreases inotropic responses to sympathetic activation. Uncoupling of the β1-adrenoceptor and the Gs-protein reduces the ability to activate adenylyl cyclase. If the ability of protein kinase A to phosphorylate L-type calcium channels is impaired, then calcium influx into the cell is reduced, leading to a smaller release of calcium by the sarcoplasmic reticulum. Reduced calcium release impairs excitation-contraction coupling, decreasing inotropy.
Revised 12/7/2022
 Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021)
Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021) Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)
Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)